Articles from Milford Molecular Diagnostics Laboratory
Sin Hang Lee, M.D., director of Milford Molecular Diagnostics Laboratory in Milford, Conn., announces that the Borrelia burgdorferi spirochetes invading the blood stream of the patients at the early localized stage of Lyme disease have duplicate flaB gene paralogs in their chromosome. For Sanger sequencing-based diagnosis of Lyme disease spirochetemia, the flaB gene with its paralogs is a more sensitive chromosomal target than the 16S rRNA gene aiming to detect a single Borrelia burgdorferi bacterium in venous blood specimens, as reported in an article published in Frontier in Bioscience-Scholar https://www.imrpress.com/journal/FBS/17/2/10.31083/FBS31280/htm
By Milford Molecular Diagnostics Laboratory · Via Business Wire · July 10, 2025

Sin Hang Lee, MD, director of Milford Molecular Diagnostics Laboratory announced today that his high complexity CLIA-certified laboratory is offering a diagnostic test directly to patients and their healthcare providers, a test that can determine all known COVID-19 variants of concern and interest, including the Omicron and Delta strains, the most predominant strains in the United States. Milford, Connecticut based Molecular Diagnostics Laboratory is believed to be the only laboratory in the United States offering this test directly to patients and their health care providers.
By Milford Molecular Diagnostics Laboratory · Via Business Wire · January 3, 2022
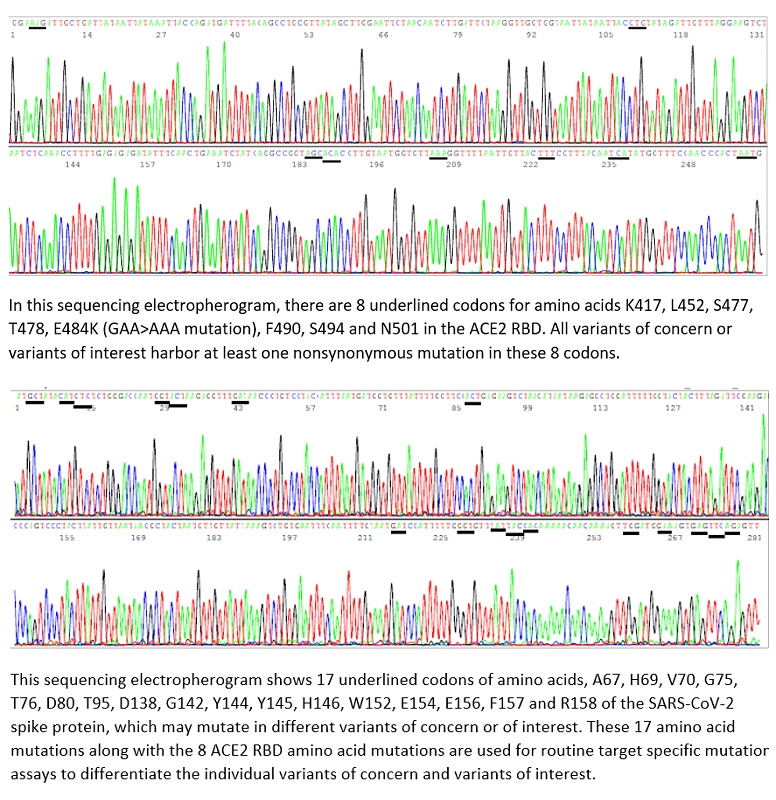
Currently circulating in the United States, the SARS-CoV-2 variants of concern are the Alpha, Beta, Gamma and Delta, and the variants of interest are the Epsilon, Eta, Iota, Kappa and Lambda. The Delta variant makes up 83% of new COVID-19 cases, said Dr. Sin Hang Lee, director of Milford Molecular Diagnostics, summarizing recently published sequence-based surveillance data.
By Milford Molecular Diagnostics Laboratory · Via Business Wire · July 29, 2021

The current Coronavirus chaos is largely caused by phony PCR tests marketed as RT-qPCR assays, explained Dr. Sin Hang Lee, director of Milford, Connecticut-based Milford Molecular Diagnostics (www.dnalymetest.com), in an article titled “qPCR is not PCR, Just as a Straightjacket is not a Jacket - The Truth Revealed by SARS-CoV-2 False-Positive Test Results.” https://researchinfotext.com/article-details/qPCR-is-not-PCR-Just-as-a-Straightjacket-is-not-a-Jacket-the-Truth-Revealed-by-SARS-CoV-2-False-Positive-Test-Results (the Article).
By Milford Molecular Diagnostics Laboratory · Via Business Wire · June 21, 2021
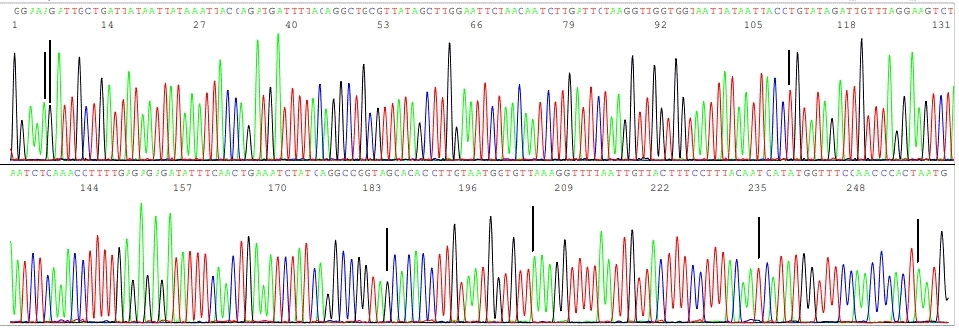
Sin Hang Lee, MD, director of Milford Molecular Diagnostics Laboratory, announced today that his CLIA-certified laboratory has been granted a permit to perform diagnostic testing for SARS-CoV-2 by nested RT-PCR followed by DNA sequencing on nasopharyngeal swab samples and residues of RNA extracts previously tested by RT-qPCR assays. The CLIA certification was officially granted on April 29, 2021 through the State of Connecticut’s Department of Public Health. Milford Molecular Diagnostics Laboratory now becomes the sole CLIA certified laboratory in the nation to offer COVID-19 RNA testing at this level of accuracy to the general public.
By Milford Molecular Diagnostics Laboratory · Via Business Wire · May 5, 2021
